Abstract
Background One-half of persons with acute myeloid leukaemia (AML) have normal cytogenetics at diagnosis and a good prognosis. However, some relapse. Whether the clonal trajectory in these persons is like that of persons with unfavorable cytogenetics at diagnosis who relapse is unknown.
Aims Interrogate the mutation topography and clone trajectory of adults with AML and normal cytogenetics at diagnosis achieving complete remission and relapsing after intensive chemotherapy (N=17), an allotransplant (N=4) or both (N=2).
Methods We performed whole exome sequencing (WES) on diagnosis (N=23), remission (N=23) and relapse samples (N=25) from 23 subjects with AML and normal cytogenetics at diagnosis who relapsed after intensive chemotherapy with (N=6) or without an allotransplant (N=17) and from 6 normal transplant donors. For filtering germline polymorphisms corresponding remission samples with corresponding donor samples were controls. Sequencing libraries were generated using Agilent SureSelect Human All Exon® kit V5(Agilent Technologies, Santa Clara, CA, USA). Mean sequencing depths were 200X (range, 145-265X) for the relapse samples and 138X (range, 106-173X) for the remission and donor samples. Median coverage was 99.9% (range, 77.6-99.9 %). NPM1, FLT3-ITD and CEBPA were also tested by standard techniques.
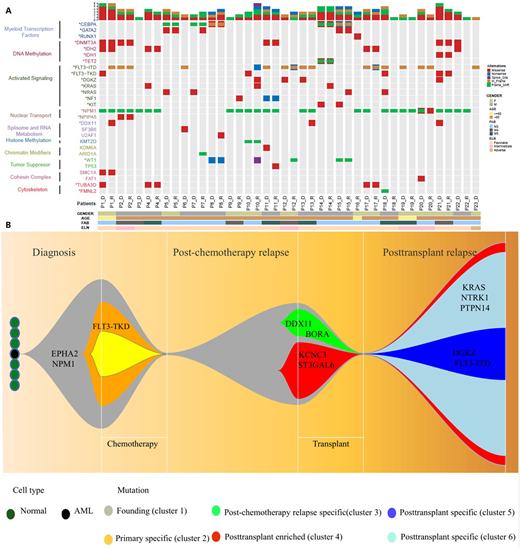
Results We detected 1317 non-synonymous somatic variants in 988 genes including 569 variants in 483 genes at diagnosis and 748 in 665 genes at relapse. 160 of these were concordant. Median numbers of mutations were both 23 (ranges,7-113 and 2-146). Most mutations were mis-sense including 84% (95% confidence interval [CI], 81, 87%)at diagnosis and 83% (80, 86%)at relapse. C>A/G>T transversions were the most common mutations at diagnosis and relapse. Frequency at relapse (52% [36, 38%]) was significantly higher than at diagnosis,37% (80, 86%; P=0.0405). This was also so for C>T/G>A transitions (24% [23, 25%] vs 21% [20, 22%]; P=0.074). Mutations in FLT3-ITD, NPM1, CEBPA, DNMT3A, IDH2, WT1 and GATA2 were frequent and typically concordant in diagnostic and relapse samples. However variable allele frequencies (VAFs) were significantly higher in relapse compared with diagnosis samples (median 41% [range, 4-86%] vs. 9% [range, 2-65%]; P<0.001). We also identified 23 novel mutated genes such as TUBA3D, MED16, PARP4 and ZADH2. There was also a significant increase in copy number variants (CNV) between diagnosis(N=17) and relapse (N=42) samples of which only 4 were shared. Mutation trajectories from diagnosis to remission to relapse were classified as: (1) similar spectrum (N=6); (2) evolution from a dominant clone at diagnosis (N=13); and (3) evolution from a minor clone at diagnosis (N=4).
Conclusions Our data illustrate the mutation topography and clonal trajectories of adults with normal cytogenetics AML from diagnosis to relapse, identify 23 new mutated genes and indicate mutation-related relapse patterns. We suggest mutation(s) possibly caused by pretransplant conditioning contribute to posttransplant relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal